The Hydrogen Challenge – Measurement Solutions for an Energy Revolution
14. January 2025

Hydrogen is at the heart of the energy transition and offers unique potential for decarbonising industry and transforming our energy consumption. However, the gas, which is often seen as one of the pillars of the energy future, poses major technical challenges, particularly in terms of storage, transport and measurement. In this article, I address the main issues associated with hydrogen and show why KELLER sensors are designed to meet the requirements.
What Exactly Is Hydrogen?
Hydrogen, the first element in the periodic table, is represented by the symbol «H». It is the most common element in the universe, with around 75 % of visible matter consisting of hydrogen. Under normal conditions, hydrogen is a colourless and odourless gas, about 14 times lighter than air. The energy density of 120-140 MJ/kg is remarkably high, which makes the element a promising energy carrier.
A distinction is made between two forms – the single hydrogen atom (H) and molecular hydrogen (H₂).
H = Single hydrogen atom
A hydrogen atom consists of a proton and an electron.
A single hydrogen atom is very reactive and is rarely found in nature. The element quickly combines with other atoms to achieve a stable configuration.

H2 = Molecular hydrogen (also called dihydrogen)
Two hydrogen atoms together form a molecule (H2).
Hydrogen occurs more frequently in nature in this gaseous form.
Hydrogen as a Fuel
Hydrogen is considered an environmentally friendly fuel, as no harmful exhaust gases such as CO₂, nitrogen oxides or particulate matter are released when it is used to generate energy. Instead, the only by-product is water – a significant advantage over fossil fuels.
Hydrogen also has some disadvantages. As the element is rarely found in its pure form in nature, it usually has to be produced first. This requires a great deal of energy and drives up costs. So-called «green hydrogen» in particular is expensive, as it is obtained exclusively from renewable energy sources such as solar and wind energy. Other types of hydrogen such as «blue» and «grey» hydrogen, on the other hand, are produced using fossil fuels, which reduces the environmentally friendly advantage.
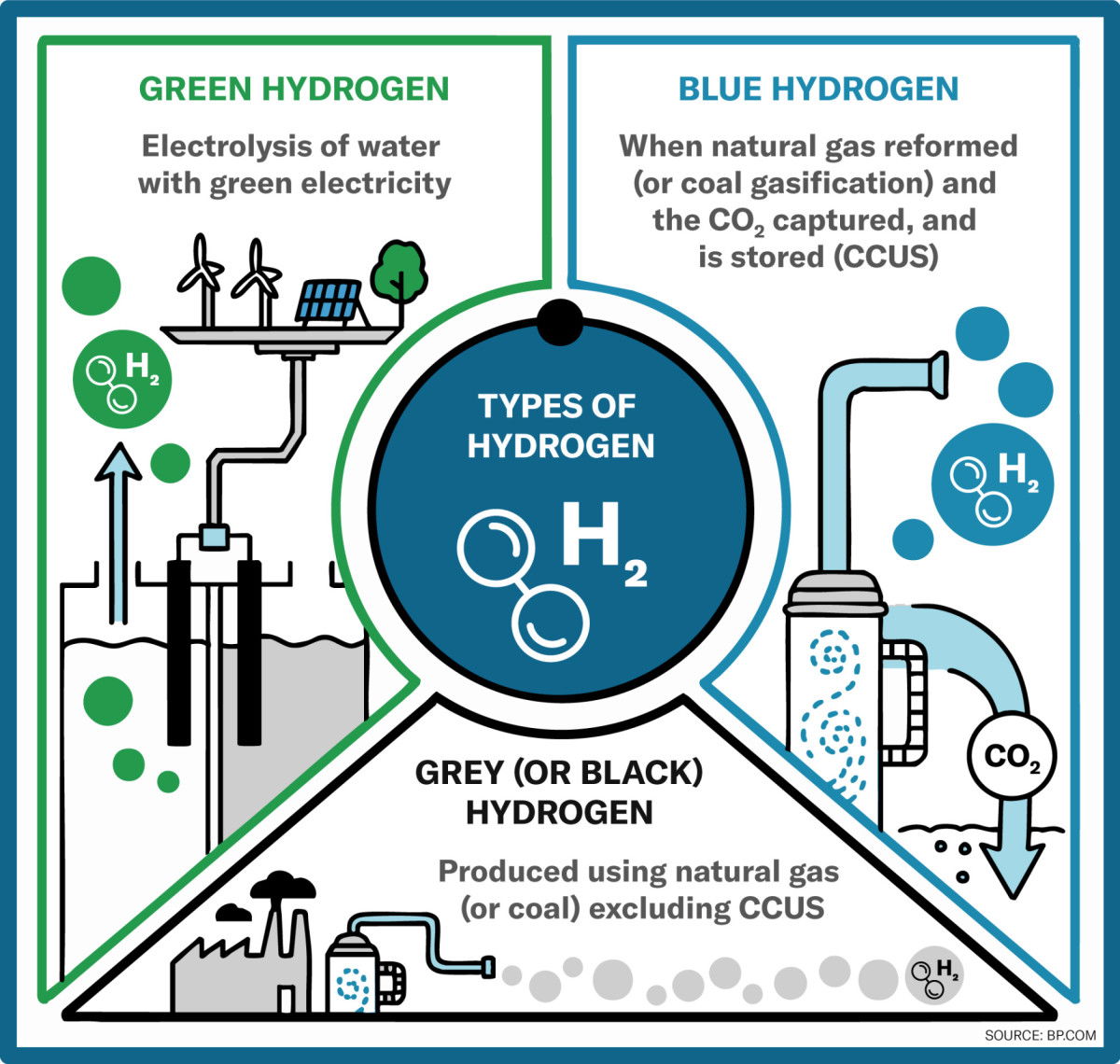
Types of hydrogen
Storage and Transport of Hydrogen
Storing and transporting hydrogen is technically demanding and poses particular challenges. As hydrogen gas is volatile and highly flammable, it must be stored and transported either under very high pressure or at extremely low temperatures. This compensates for the low energy density per volume. These conditions place high demands on the safety and technical equipment of the storage systems.
Comparison of hydrogen consumption and drum capacity
A light car needs around 7 kg of hydrogen to cover a distance of 700 kilometres. A bottle of Lemonade with a capacity of 750 ml, for example, can hold less than 70 milligrams of hydrogen at atmospheric pressure.
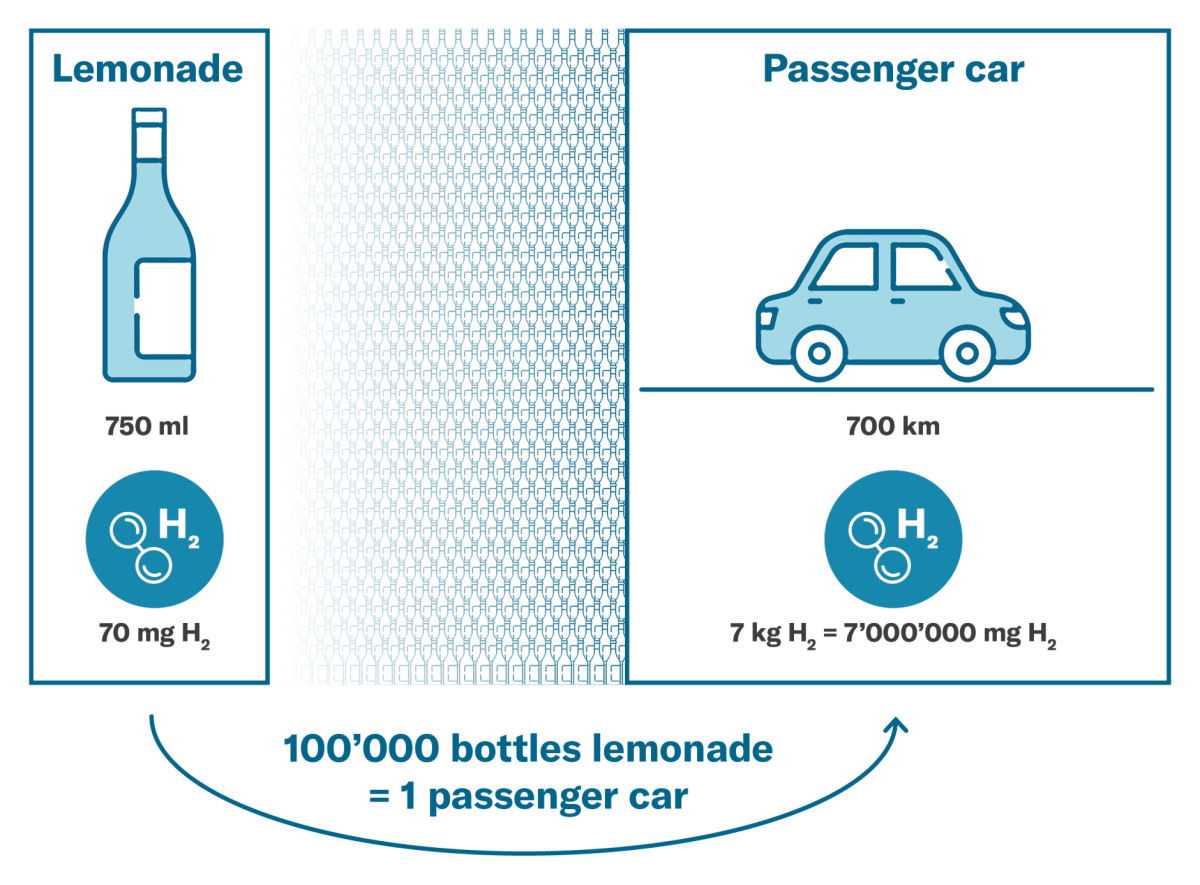
Hydrogen consumption and drum capacity
Various methods of storing hydrogen make it possible to fulfil the needs of our customers:
- Gaseous hydrogen: Hydrogen can be stored in gaseous form in special tanks by high-pressure compression to up to 700 or even 950 bar. However, this type of storage requires particularly robust and expensive tanks.
Gaseous hydrogen tanks come in various types, ranging from heavy metallic models (Type I) to ultra-lightweight composites (Type IV). Gravimetric ratios range from 1-2% for metallic tanks to 5-10% for high-pressure composites, which are ideal for vehicles. Type V tanks, still under development, promise even better performance.
This compressed hydrogen can also be transported via pipelines to supply large hydrogen consumers and refuelling stations. - Liquid hydrogen: An alternative approach is the liquefaction of hydrogen. This requires extremely low temperatures of around -252 °C, which requires a high energy input. In addition, transport in tankers or cryogenic tanks requires extreme thermal insulation and a special infrastructure.
- Alternative methods: Another possibility is to combine hydrogen with other elements. So-called «products» are created that are easier to transport. Intensive research is currently being conducted into the methanisation of hydrogen, in which hydrogen is converted into methane. Existing natural gas infrastructures can be used for this. Other products such as methanol (CH30H) and ammonia (NH3) are also being investigated for the transport of hydrogen or energy. Projects with environmentally friendly ammonia, for example, are a key area for applications in industrial production and transport.
Solid hydrogen storage, based on materials such as metal hydrides or innovative compounds like complex hydrides and nanoporous materials, offers a compact and secure solution for ultra-light mobility or maritime applications, including boats and submarines. These technologies enable high-density hydrogen storage without extreme compression.
The market for the storage of gaseous, liquid or geological hydrogen is booming. The innovations are aimed at the energy conversion industries for transporting hydrogen by pipeline or tanker, whether by road or sea.

Transport of hydrogen by pipeline
Pressure Transmitters and the Challenges of Hydrogen Measurement
The accurate and reliable measurement of hydrogen is crucial for industrial applications. When measuring the pressure of the element, special requirements are placed on the measuring instruments. Thanks to their advanced technology, our sensors are able to overcome the unique challenges of this gas:

Hydrogen embrittlement
In the phenomenon of hydrogen embrittlement, hydrogen penetrates the structure of the metal and changes its properties. The changes can become noticeable through small cracks and can even lead to fractures and material failure.
To counteract this effect, we use materials that are less susceptible to hydrogen embrittlement, e.g. the stainless steel alloy (AISI 316L / 1.4435) with a nickel content of 14%.
Permeation
Permeation describes the process by which hydrogen penetrates through a layer of a material. Hydrogen normally occurs in bound, molecular form as H2 (pure hydrogen) or H2O (bound with oxygen as water) and only rarely as a single hydrogen atom (H). During electrolysis or thermal shock (glossary term or link to Wikipedia), hydrogen molecules (H2) can separate and become hydrogen atoms (H). Due to their lightness, individual hydrogen atoms (H) can penetrate a metal lattice and make their way into the metal membrane. This process can be divided into the following three steps:
- Hydrogen is absorbed by the material.
- Hydrogen penetrates the material by diffusion (H atoms penetrate steel and bind directly back into H2 molecules after penetration).
- Hydrogen escapes on the other side of the material.
The duration of this process is called the permeation rate. To reduce the permeation of hydrogen, the membranes are coated with a protective layer of gold. Gold has a lower permeation rate than steel. This means that gold acts as a barrier and extends the diffusion time many times over.
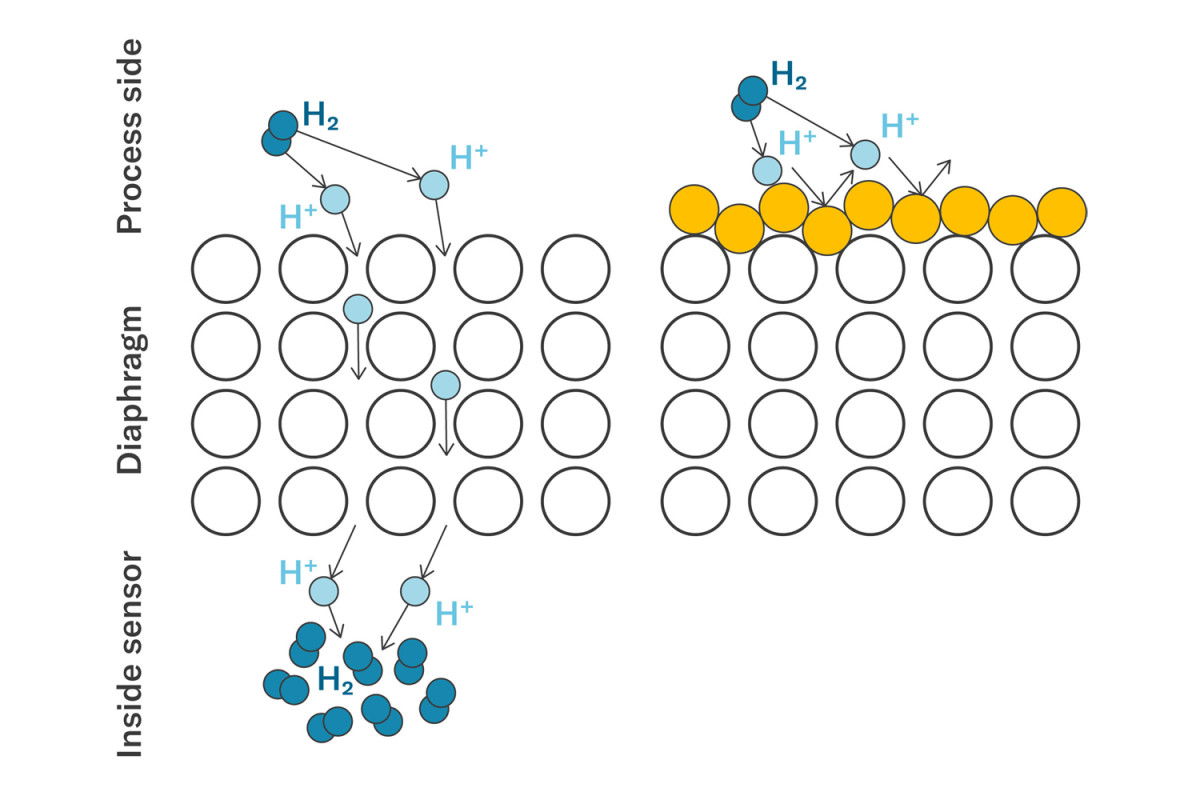
Permeation process: Hydrogen penetrates through a layer of material.

Leakage
The combination of hydrogen and oxygen in the air can create a highly explosive mixture. According to the principle of the fire triangle, which defines three essential elements of combustion, it is crucial to prevent hydrogen (the fuel) from escaping.
The fully welded design without internal seals and the metal-sealing process connections minimise the risk of leakage. We believe that elastomer seals pose a significant risk of leakage and are therefore excluded from the design.

ATEX-certified and intrinsically safe
For additional protection under extreme conditions, our pressure transmitters are ATEX-certified. KELLER series, labelled with the additional identification feature «H2», are also available in the intrinsically safe version, labelled with «Ei». This means that sensors can be used in potentially explosive atmospheres.
Overview of Hydrogen Sensors
Our Sensors for Hydrogen Applications
Our portfolio for hydrogen applications continues to grow with products that fulfil demanding safety and performance standards. The solutions cover different needs across the entire hydrogen supply chain - be it for refining, green ammonia production, metallurgy, storage, transport or electrolysis.
Our hydrogen application page provides an overview of everything we have to offer for H2 applications: www.keller-druck.com/h2









